Dual MoA inhibiting DNMT1 and DNA repair induces strong efficacy
Stable PK profile can be achieved by evading the metabolic enzymes of Decitabine
NTX-301 is a nucleoside-based DNMT1 inhibitory targeted anticancer therapy for various blood cancers/solid cancers caused by abnormalities in DNA methyl-transferase (DNMT) that regulates the gene expression patterns in our body. The indications are MDS (myelodysplastic syndrome)/AML (acute myeloid leukemia) among blood cancers, and ovarian and bladder cancers among solid cancers.
Unlike the existing DNMT1 inhibitors, which show efficacy only in blood cancers, NTX-301 shows excellent efficacy in various solid cancers.
MDS/AML have high incidence rate in the older adults and have limited treatment options. The 5-year survival rate from diagnosis is less than 15%.
Ovarian/bladder cancers have serious issue of developing resistance to platinum-based chemotherapy. Once resistance occurs, the survival rate is extremely low.
NTX-301 overcomes resistance mechanisms of competing DNMT1 inhibitors through the Thio- modification in the nucleoside structure
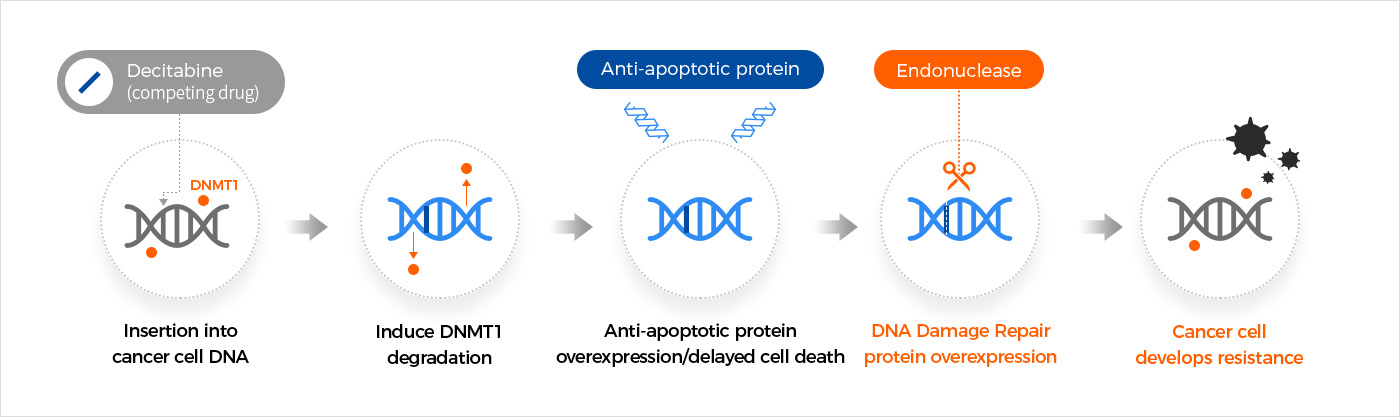

Dual MoA inhibiting DNMT1 and DNA repair induces strong efficacy
Stable PK profile can be achieved by evading the metabolic enzymes of Decitabine
Cancer-selective activation feature leads to improved safety profile
Increased safety margin allows higher dosage and effective treatment compared to existing DNMT1 inhibitors
Oral administration is easier for patients to take the drug
| Project | Indication | Development Stage Discovery
Preclinical
Phase 1
Phase 2
Phase 3
|
Clinical Site |
|---|---|---|---|
| NTX-301 | Solid tumor | - US Phase 1b/2a(ongoing) - AUS Phase 1a(finished) |
|
| Hematological Malignancy (MDS/AML/CMML) |
- US Phase 1a(ongoing) |